Authors:
RK Neelakandan - Health Quality and Safety Engineering lead
Teginder Singh - Global Head of Regulatory and Safety Operations
Nikita Holland - Principal Architect, Medtech and Digital Health Solutions
Key Takeaways
- The life sciences industry's shift from heavyweight Computer System Validation (CSV) to a leaner, risk-based Computer Software Assurance (CSA) model, coupled with the rapid adoption of AI, renders traditional on-premise validation methodologies increasingly inefficient and unsustainable..
- Google Cloud provides the necessary infrastructure for a CSA-based approach, reducing qualification burdens through its shared fate model and inheritable controls. Additionally, it enables automated, auditable testing via Infrastructure as Code (IaC) and CI/CD pipelines.
- Vertex AI enables the creation of a compliant "AI factory" for GxP use cases, with built-in traceability for experiments, governed data sources in BigQuery, version-controlled model deployment, and continuous monitoring to manage model drift.
- This convergence allows organizations to innovate with AI-powered GxP systems while maintaining the highest standards of patient safety and data integrity, directly realizing the efficiency goals of CSA.
Introduction
For decades, GxP validation has been anchored in the deliberate, paper-heavy world of Computer System Validation (CSV). It gave us structure and control. But today, a perfect storm is brewing. The FDA is championing a move to a leaner, risk-based Computer Software Assurance (CSA) model, while the industry itself, guided by frameworks like the ISPE GAMP® AI Guide, is racing to adopt the transformative power of Artificial Intelligence.
This convergence creates a critical inflection point. The rigid, "validate-once" methodologies that defined the past are fundamentally incompatible with the dynamic, data-driven intelligent systems of the future that continuously infer from new data, intelligent systems of the future. The question is no longer if we will adopt these new paradigms, but how we can do so while maintaining the unwavering commitment to patient safety and data integrity that defines our industry.
The answer lies not in upgrading our on-premise servers, but in fundamentally transforming our approach. The answer is cloud computing.
The Breaking Point: Why Traditional Validation Can't Keep Pace
The CSV model, for all its strengths, was built for a different era—an era of static, monolithic software. When we apply its principles to the modern GxP landscape, the cracks begin to show.
- The CSA Challenge: The FDA's CSA guidance encourages us to move away from exhaustive, "tick-box" testing and towards critical thinking. It asks us to focus assurance efforts on what truly matters: high-risk functions that impact patient safety. In a traditional on-premise environment, this agility is difficult. Provisioning validated test environments can take weeks, manual testing remains the default, and the documentation burden for even minor changes can be immense.
- The AI Imperative: As the ISPE GAMP AI Guide details, AI/ML systems are a different species of software. They are:
- Probabilistic, Not Deterministic: Their outputs are predictions, not certainties.
- Data-Dependent: The model is only as good as the "Fit for Purpose Data" it's trained on. Data governance isn't an afterthought; it's the foundation.
- Dynamic: Models are designed to evolve and adapt. This includes phenomena like 'model drift' (where performance degrades over time due to changes in real-world data), as well as planned updates through retraining, fine-tuning, or adjustments to data inputs (like Retrieval Augmented Generation - RAG). Such systems require continuous monitoring and assurance throughout their operational lifecycle, not just a one-time validation..
The traditional static CSV framework, conceived for an era of monolithic, slowly updated on-premise systems, is fundamentally ill-suited for the dynamic, continuously evolving nature of modern machine learning. CSV's process-laden approach emerged from a time when technology acquisition and deployment took weeks or months, allowing ample time for extensive, document-heavy validation. In contrast, cloud environments offer near-instantaneous provisioning and dynamic scaling, where changes can occur in seconds or minutes. Applying static CSV to such agile systems creates an insurmountable bottleneck. Validating a machine learning model, therefore, cannot be a one-time event; its performance and fitness for purpose require continuous assurance that matches the speed and dynamism of cloud operations.
Building a Foundation for CSA on Google Cloud
Before we can run, we must walk. The move to CSA for all computerized systems requires a new kind of approach to infrastructure management—one that is inherently flexible, automated, and auditable. This is where Google Cloud excels, not just by providing the infrastructure, but by enabling organizations to take full advantage of its capabilities to streamline assurance.
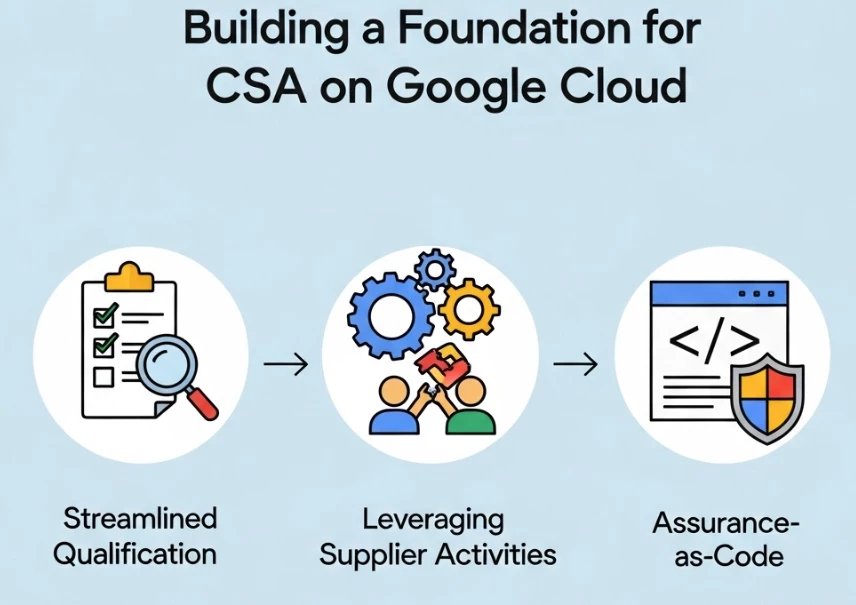
- Streamlined Qualification & Leveraging Supplier Activities: Under the cloud's shared fate model, Google provides comprehensive compliance documentation. You can leverage Google Cloud's extensive portfolio of compliance reports (e.g., SOC 2/3, ISO/IEC series, HIPAA attestations) as foundational evidence for your vendor assessment and system qualification activities. This directly aligns with the CSA principle of leveraging supplier documentation, dramatically reducing your qualification burden and allowing you to focus your assurance efforts on the application layer and its specific intended GxP use...
- Assurance-as-Code: The CSA framework encourages robust, agile testing. With tools like Infrastructure as Code (IaC) and CI/CD pipelines on Cloud Build, our validation strategy becomes code. You can define, version-control, and automatically deploy identical, qualified test environments in minutes, not weeks. The 'record' of your testing is no longer a collection of screenshots, but an immutable, timestamped execution log from a CI/CD pipeline. This makes the unscripted and exploratory testing favored by CSA not only possible but repeatable and auditable. While the 'Assurance-as-Code' approach, with its immutable CI/CD logs, IAM-controlled deployments, and version-controlled artifacts, provides a robust framework for meeting the electronic record aspects of 21 CFR Part 11, specific digital signature requirements for formal approvals may still necessitate integration with a dedicated digital signing service or a documented process leveraging strong user authentication.
The GxP AI Factory: Enabling Compliant AI with Vertex AI
Adopting AI in a GxP environment isn't about hiring a few data scientists; it's about building a compliant, end-to-end "AI factory." The ISPE GAMP AI Guide provides the blueprint, and Google Cloud's Vertex AI provides the integrated MLOps (Machine Learning Operations) platform to build it.
Vertex AI is a unified environment that provides the traceability and control that regulators demand at every stage of the AI lifecycle.
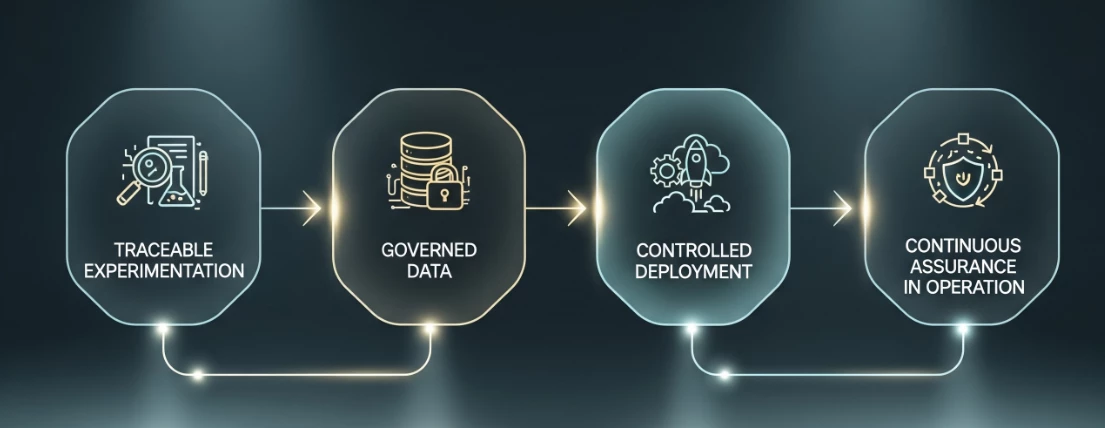
- Traceable Experimentation: During the Project Phase, data scientists can use Vertex AI Workbench for model development. Crucially, every training run—every dataset version, hyperparameter, and piece of code—can be tracked as a distinct run in Vertex AI Experiments. This creates an indelible audit trail, connecting the final model directly back to its development lineage.
- Governed Data: The concept of "Fit for Purpose Data" is central to the ISPE guide. BigQuery, Google's serverless data warehouse, serves as the single source of truth for your training and test data. With granular Identity and Access Management (IAM) and data lineage capabilities, you can ensure that only the right people have access to the right data for the right reasons.
- Controlled Deployment: Once a model is ready, it's not just copied to a server. It is registered in the Vertex AI Model Registry. This provides a central, version-controlled repository for all your GxP models. You can clearly distinguish a "development" version from a "QA-approved" or "production-released" model, ensuring only validated artifacts are deployed.
- Continuous Assurance in Operation: This is the game-changer. The ISPE guide rightly highlights the risk of "model drift." Vertex AI Model Monitoring provides the solution. Once a model is deployed, you can automatically monitor its predictions for skew and drift. When performance deviates from the established validation baseline, it can trigger an alert, initiating a formal quality process (e.g., a CAPA or change control). This isn't periodic review; it's continuous, automated assurance of the model's validated state.
Putting It All Together: A CSA-AI Approach for GxP Processes
Let's move from theory to a practical Quality System application, illustrating how a CSA-AI approach transforms a common GxP challenge. Imagine a medical device manufacturer struggling with recurring non-conformances. The traditional CAPA (Corrective and Preventive Action) process is often slow and resource-intensive, relying on manual data review across disparate systems to identify true root causes.
However, under CSA principles, a system designed to accelerate insights for root cause analysis—rather than automating the final quality decision—would typically be classified as a 'not-high-risk' function. Its failure would result in a slower, less effective investigation, not an immediate risk to product quality or patient safety. This is a perfect scenario where a CSA-AI approach on Google Cloud can revolutionize efficiency.
With a CSA-AI approach on Google Cloud, the entire process is transformed:
- The Solution: An AI-powered "Root Cause Intelligence Tool" is developed. Production data, equipment logs, and batch records are consolidated into BigQuery. Unstructured text from deviation reports and engineer notes is processed using Vertex AI's Natural Language Processing (NLP) APIs to identify trending keywords and hidden correlations that human investigators might miss.
- The Assurance (CSA in Action): Instead of a heavyweight CSV package, the manufacturer applies a lean, critical-thinking approach to assurance. The validation strategy focuses on unscripted, exploratory testing led by experienced Quality engineers. They "challenge" the system with known historical CAPAs to confirm it correctly surfaces the known correlations. The focus is on verifying the tool's utility as a powerful analytics aid, not on documenting every possible query.
- The Record: The validation record is lean and digital. It documents the intended use, the risk analysis classifying the tool as "not high risk," and a summary of the successful exploratory testing. This approach delivers a powerful, validated tool to the Quality team in a fraction of the time and cost of a traditional CSV project, directly realizing the efficiency goals of the CSA framework.
Conclusion: The Future is Assured, Not Just Validated
The path forward for MedTech is undeniably clear: to embrace the agility demanded by CSA and the transformative power of AI, we must move beyond the limitations of past methodologies. This represents more than a technology upgrade; it's a fundamental paradigm shift in how we approach quality and compliance. It necessitates a parallel evolution in organizational culture, fostering deep collaboration between Quality Assurance, IT, and data science teams under a shared vision of continuous, automated assurance.
Google Cloud provides the robust, foundational platform for this essential transformation, offering the integrated tools required to streamline CSA and build the compliant, traceable, and continuously monitored AI systems that will define the next generation of GxP innovation. The strategic imperative for our industry is no longer 'Can we achieve GxP in the cloud?' but rather, 'How can we possibly achieve the future of GxP without it?


