Blog Authors:
Bhavana Bhinder - Google Cloud, Office of CISO HCLS
RK Neelakandan - Google Health Quality and Safety Engineering Lead
Ivan Nardini - Vertex AI Developer Advocate
For leaders in the Life Sciences industry, maintaining GxP (Good x Practice) compliance is fundamental to ensuring product quality, safety, and regulatory approval. As Artificial Intelligence (AI) and Machine Learning (ML) become integral to processes from drug discovery to manufacturing, these powerful systems must also operate under strict GxP controls.
This presents a significant challenge: How can organizations innovate with AI while ensuring the rigorous documentation, traceability, and change control mandated by regulators? Traditional methods of tracking models in documents or spreadsheets are often inefficient, prone to human error, and can make audit preparations a time-consuming and stressful ordeal.
Google Cloud helps organizations navigate this complexity through our Shared Fate model. We manage a significant portion of the underlying security and compliance responsibilities, allowing your teams to focus on scientific innovation. A key component of this is the Vertex AI Model Registry, which provides the oversight and governance needed to manage the entire lifecycle, from model registration to secure deployment on an endpoint.
A Centralized Solution for AI Governance and Compliance
Vertex AI Model Registry acts as a centralized system designed to support governance and oversight for your AI models in a GxP environment.
For Life Sciences companies, it provides critical capabilities that directly support GxP principles and simplify compliance.
- A Single Source of Truth for Model Oversight: The Model Registry provides a unified dashboard view of all models, their different versions, and their current status. This gives Quality Assurance and regulatory teams clear visibility into every AI asset across the organization.
- Complete Traceability and Audit Readiness: Every time a model is updated, a new, unchangeable version is created and logged. This creates a complete, time-stamped history of every model, allowing you to instantly answer auditor questions like, "Which exact version of the model was used to analyze this batch data?"
- Robust Documentation and Change Control: The system allows for detailed documentation to be directly associated with each model version. This can include validation reports, performance metrics, and development notes. You can also apply clear labels, such as Pending-QA-Review or Approved-for-Production, to manage the model's entire lifecycle.
- Maintain Validated State of a Model: Vertex AI Model Monitoring can track and alert you when deviations surpass a predefined threshold that you set through monitoring objectives. This enables timely re-evaluate or retrain of the model to ensure it is behaving as intended.
- Secure, Role-Based Access: The platform integrates with Google Cloud's robust security controls, ensuring that only qualified and authorized personnel can approve, modify, or deploy AI models. This enforces proper segregation of duties, a core principle of GxP.
- Controlled and Reliable Model Updates: The Model Registry ensures that only fully approved and validated model versions can be deployed into operational use. If an issue is ever detected, teams can quickly and safely revert to a previously known and trusted version, ensuring system stability and patient safety.
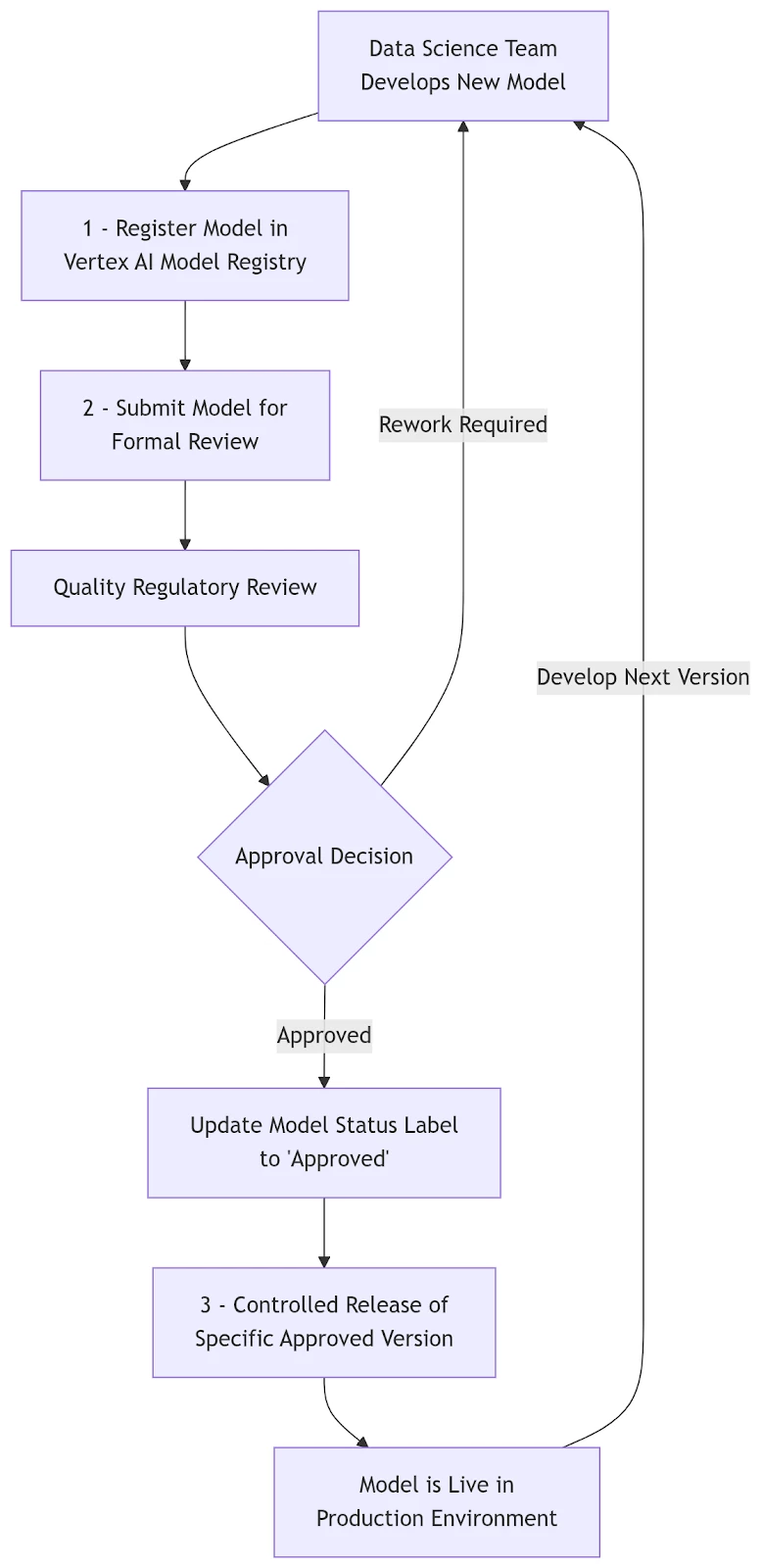
How Model Registry Enhances Your GxP Workflow
Think of the Vertex AI Model Registry as a digital vault for your AI models, where each model has a detailed logbook tracking its entire history. The process aligns seamlessly with established quality management principles.
1. Secure Registration and Versioning
When a data science team develops a new model, it is registered in the central system. This action captures a unique digital record of the model, creating the first entry in its permanent audit trail.
2. Formal Review and Approval
Once registered, the model can be formally submitted for review. Stakeholders from Quality and Regulatory affairs can access all associated documentation and performance data directly within the system. They can then apply a digital "stamp of approval" by updating the model's status label to Approved, ensuring a clear and documented approval process.
3. Controlled Release to Production
After approval, the system permits only that specific, validated version of the model to be released into the live environment. This controlled process prevents unauthorized or un-validated AI from ever being used in a regulated capacity, providing a critical GxP control point.
This entire workflow is logged and auditable, giving your organization confidence that your use of AI meets the highest standards of quality and regulatory compliance.
Partnering for Compliance and Innovation
Adopting AI in a GxP-regulated environment requires a platform built on a foundation of security, transparency, and control. Vertex AI Model Registry provides the technical capabilities to manage your AI assets in a compliant manner, reducing risk and empowering your teams to innovate faster.
To learn more about how Google Cloud supports the Life Sciences industry:
- Read Google Cloud's approach to GxP Compliance.
- Explore our Solutions for the Life Sciences Industry.
- Visit the Vertex AI platform page to discover more AI capabilities.


